“Are you N2 your tires?” asks Ingersoll Rand, marketers of Nitrogeneration™, a new way to inflate your tires…and Ingersoll Rand’s bottom line.
“Are you N2 your tires?” asks Ingersoll Rand, marketers of Nitrogeneration™, a new way to inflate your tires…and Ingersoll Rand’s bottom line.
By filling your tires with pure nitrogen instead of air, Ingersoll Rand, one of the various nitrogen-into-tires purveyors, contends that you will: (1) enhance safety because pressure decreases more slowly, (2) lengthen tire life because internal oxidation is eliminated, and (3) increase fuel economy because better inflation increases mpg.
This is terrific—and disingenuous—marketing: a very slight improvement gets touted as a revolution. (And by the look of the brochure graphic above, Nitrogeneration is very green.)
 Out goes the bad air; in goes the good. For a $25-100 charge, your neighborhood champion of good tire management will remove the commonplace air currently leaking through and eating away at your tires and replace it with (nearly) pure nitrogen. Let’s examine the claims one-by-one.
Out goes the bad air; in goes the good. For a $25-100 charge, your neighborhood champion of good tire management will remove the commonplace air currently leaking through and eating away at your tires and replace it with (nearly) pure nitrogen. Let’s examine the claims one-by-one.
It is indisputable that under-inflation is a safety hazard. But does nitrogen diffuse through tire walls less rapidly than air? What’s air? Not including water vapor (its constituent portion varies…think Tucson vs. Mobile…so more on water vapor later), air is approximately 78% nitrogen, 21% oxygen and 1% argon.
 Side note about argon: Just prior to my 50th birthday, I announced to my children that a half-century of the name Steve was enough, and I would be changing my name to Argon. My four daughters rolled their eyes both figuratively and literally; my son, nearly 13, asked for a reason.
Side note about argon: Just prior to my 50th birthday, I announced to my children that a half-century of the name Steve was enough, and I would be changing my name to Argon. My four daughters rolled their eyes both figuratively and literally; my son, nearly 13, asked for a reason.
Me (standing tall): Argon is a strong name. It is a noble gas.
Son (with gimlet eye): Yeah, pop. But it’s also inert.
I had relied upon metaphor; he, not yet the poet he is today, pointed to reality. Single at 50, and unwilling to see myself as inert, I chose to remain Steve.
There’s also a bit of carbon dioxide—that in-the-news greenhouse gas—and some other very minor constituents, but for as far as the tire is concerned, inflation with nitrogen replaces about one-fifth of the internal gas (just the oxygen). Even though oxygen, element number 8, is heavier than nitrogen, element number 7, and they both form diatomic molecules (O–O and N–N), electrostatic forces within the atoms draw the oxygen electrons in tighter (eight positively charged protons in the oxygen nucleus attract its eight negatively charged electrons with more force than does nitrogen’s seven), making the oxygen molecule slightly narrower. For this reason, oxygen will diffuse through the tire wall (which is a thick rubber membrane) more rapidly than will the larger, but lighter nitrogen molecule.
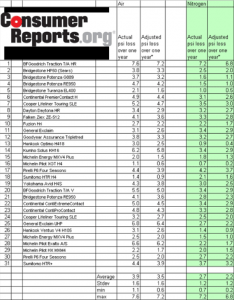
Ingersoll Rand states, “Tires filled with nitrogen maintain their pressure up to 40% longer than compressed air.” But they offer no scientific study to back up this claim. And since both air and “pure” nitrogen (Ingersoll Rand’s nitrogen-generation machine, which extracts nitrogen from ambient air only guarantees 95% nitrogen) are continuously diffusing through the tire wall, albeit at different rates, what does it mean to “maintain pressure”? Consumer Reports, however, has done some research. In 2006-07 they evaluated 31 pairs of all-season tires, filling one tire with air and the other with nitrogen, both at 30 psi (pounds per square inch). The tires were left outdoors for a year, then the inflation pressure was rechecked. The result: nitrogen does reduce pressure loss over time, but the reduction is only a 1.3 psi difference from air-filled tires…certainly insufficient to encourage owners to pass up regular inflation checks. And if one is going to check tire pressure and top off with air, why pay for nitrogen?
 The second claim is that tires last longer when not subjected to oxidation. This is undoubtedly true, but what percentage of passenger tires are replaced because of rubber fatigue caused by oxidation? I do not have data on this, but my lifetime of tire replacement and much anecdotal chuffing with other tire replacers (New York Times article here) leads me to tread wear—due to improper inflation and/or high mileage—as the overwhelming reason for tire purchases. Oxidation might be an important consideration for aircraft, space shuttle, or semi-truck tires (higher pressures, more weight, fast stops, temperature extremes, oxygen supporting combustion, etc.). Water vapor in these tires would also be a hazard because condensation in cold conditions could change pressures significantly. Are these concerns important for passenger cars travel? Unlikely.
The second claim is that tires last longer when not subjected to oxidation. This is undoubtedly true, but what percentage of passenger tires are replaced because of rubber fatigue caused by oxidation? I do not have data on this, but my lifetime of tire replacement and much anecdotal chuffing with other tire replacers (New York Times article here) leads me to tread wear—due to improper inflation and/or high mileage—as the overwhelming reason for tire purchases. Oxidation might be an important consideration for aircraft, space shuttle, or semi-truck tires (higher pressures, more weight, fast stops, temperature extremes, oxygen supporting combustion, etc.). Water vapor in these tires would also be a hazard because condensation in cold conditions could change pressures significantly. Are these concerns important for passenger cars travel? Unlikely.
The third claim is that correctly inflated tires yield increases gas mileage. True. So check your tires. Then fill them. Nuff sed.
 Some people, however, need a change of color on their beer label to inform them when their brew is cold enough to drink.
Some people, however, need a change of color on their beer label to inform them when their brew is cold enough to drink.
For them, I recommend nitrogen-filled tires.